Muscle atrophy is of great medical concern, not only for astronauts in space but also for immobilized patients or elderly people on earth. Random Positioning Machines (RPM) are ground based devices frequently used to expose cells to simulated microgravity. The RPM consists of two gimbal mounted frames which rotate constantly to average the earth gravity vector over time to zero. Many studies on myoblasts have been carried out to study muscle development but only a few data are available concerning myoblast differentiation under simulated microgravity. The goal of this project was to improve the means of cell cultivation on the RPM and to study myoblast differentiation under simulated microgravity.

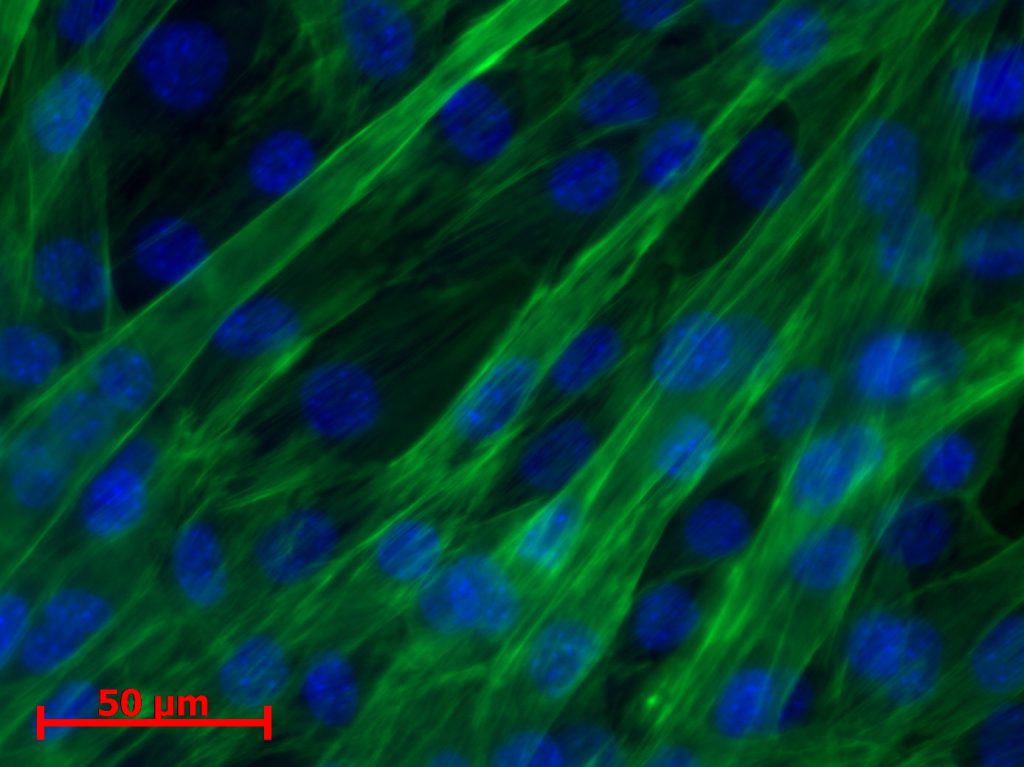
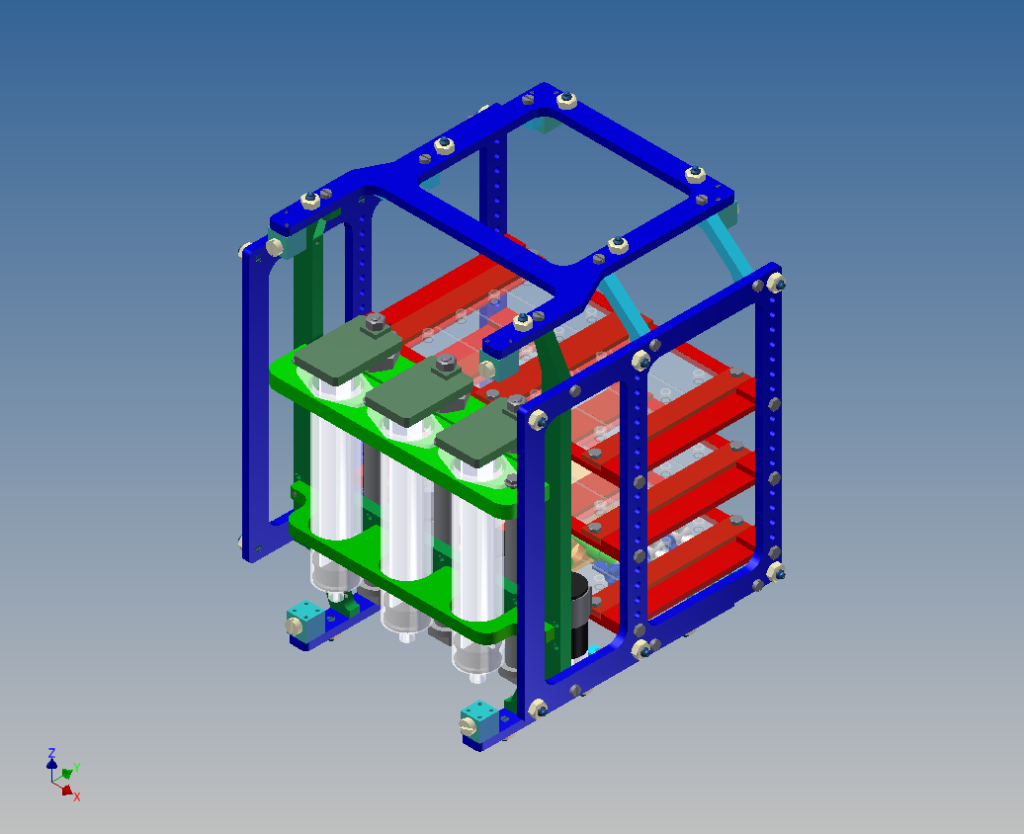
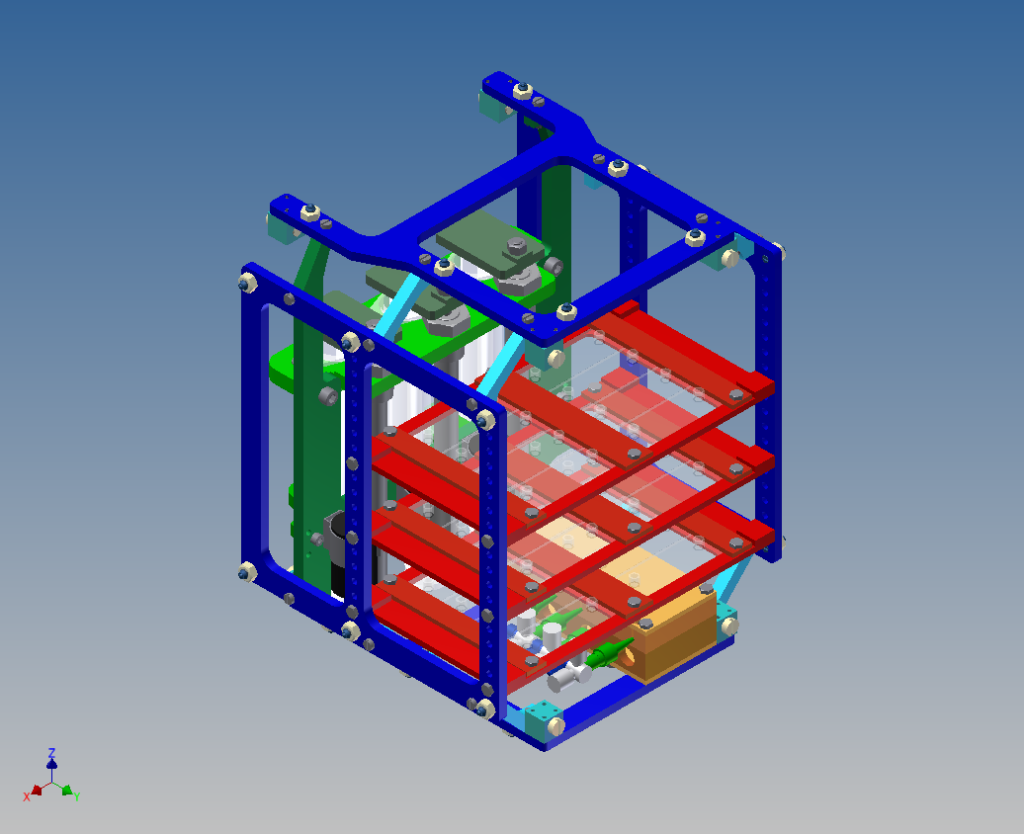
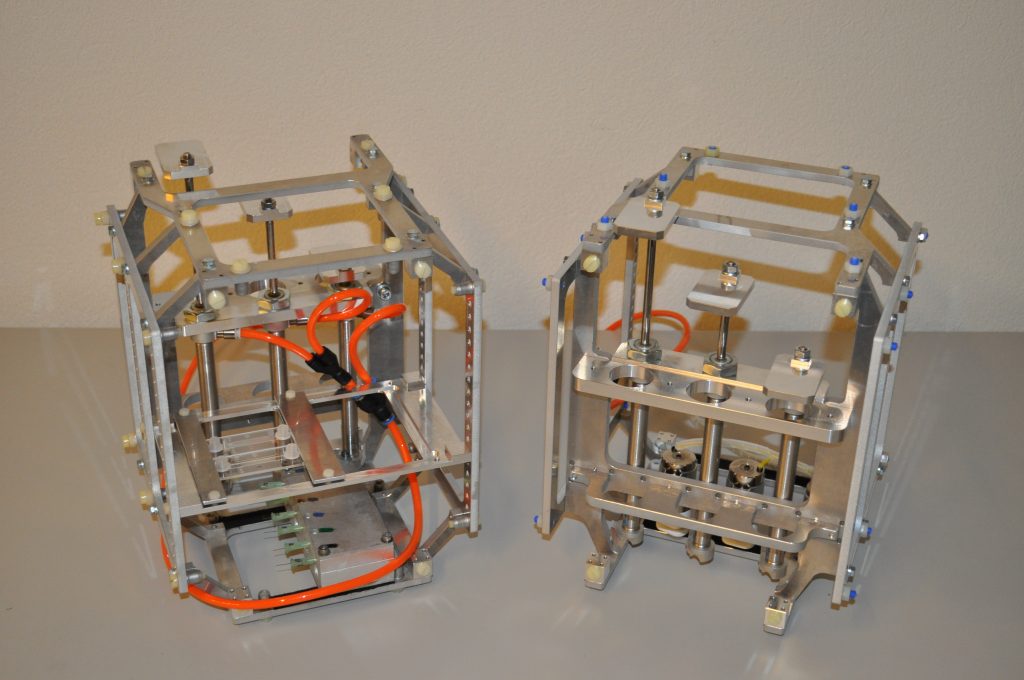
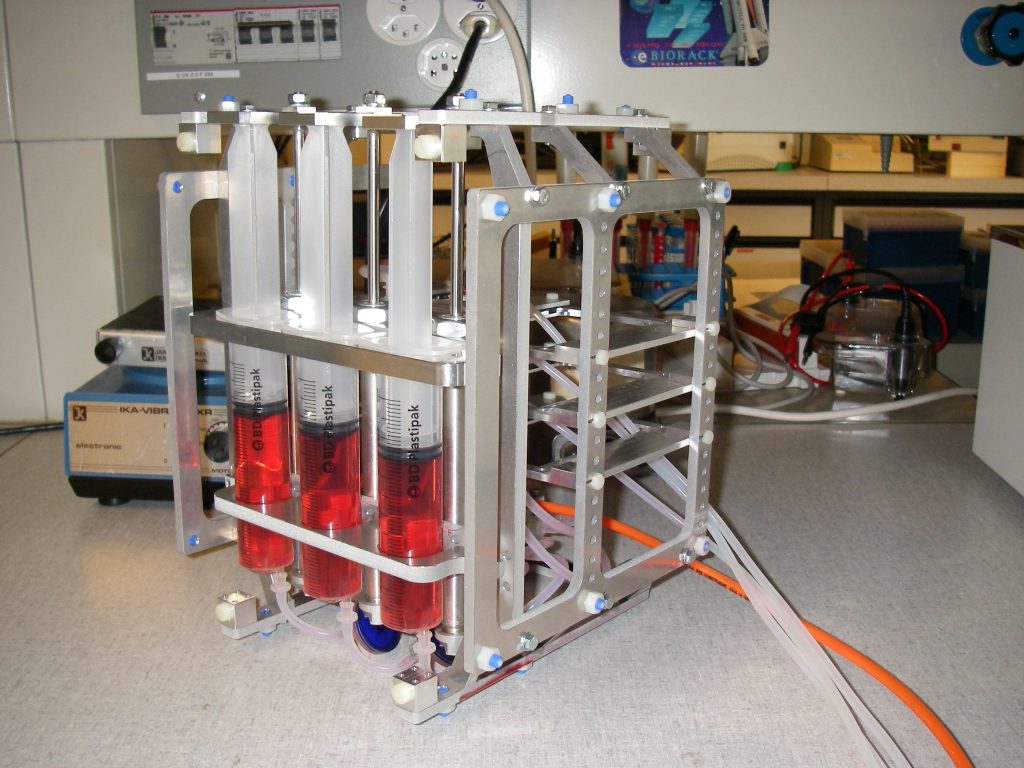
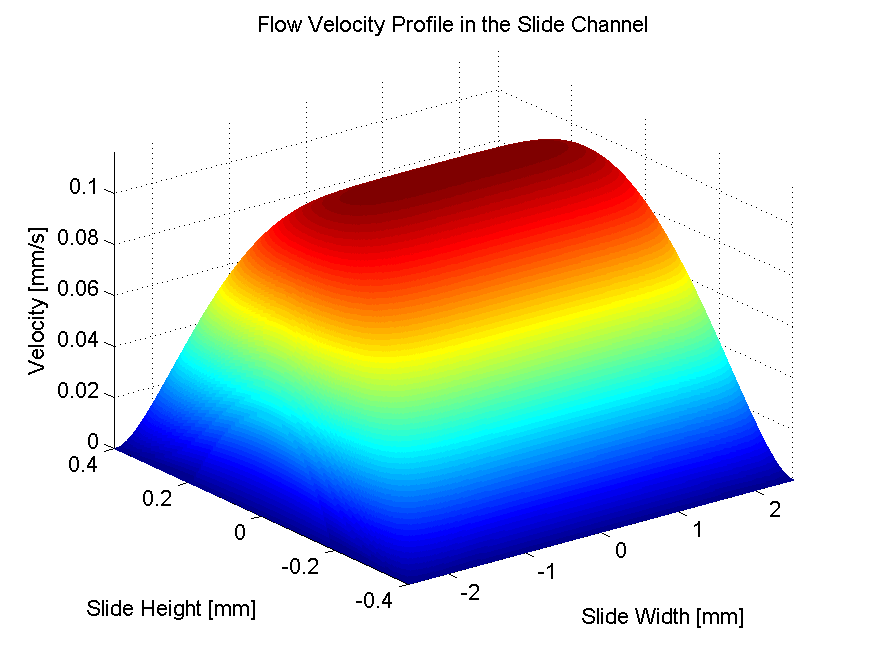
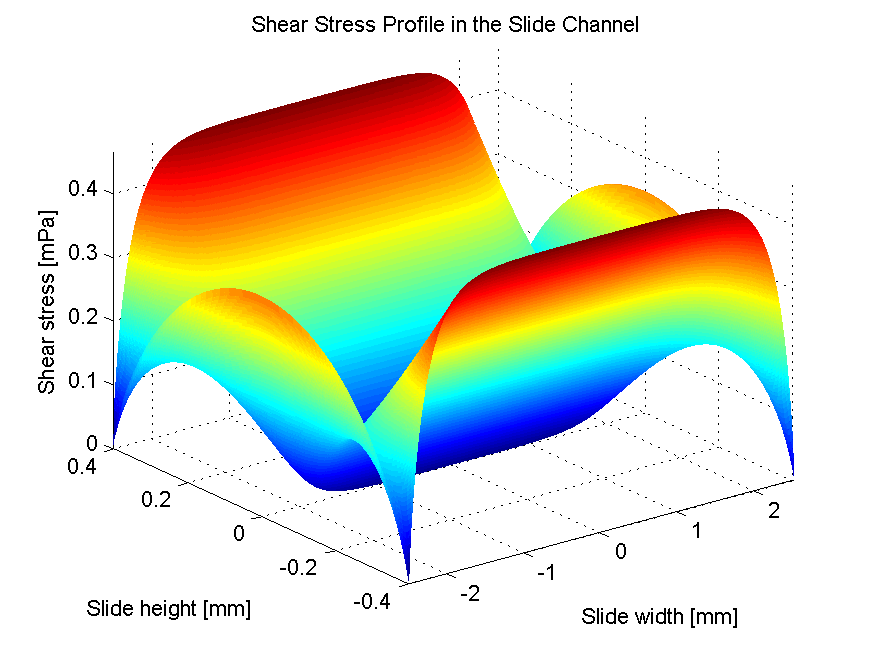
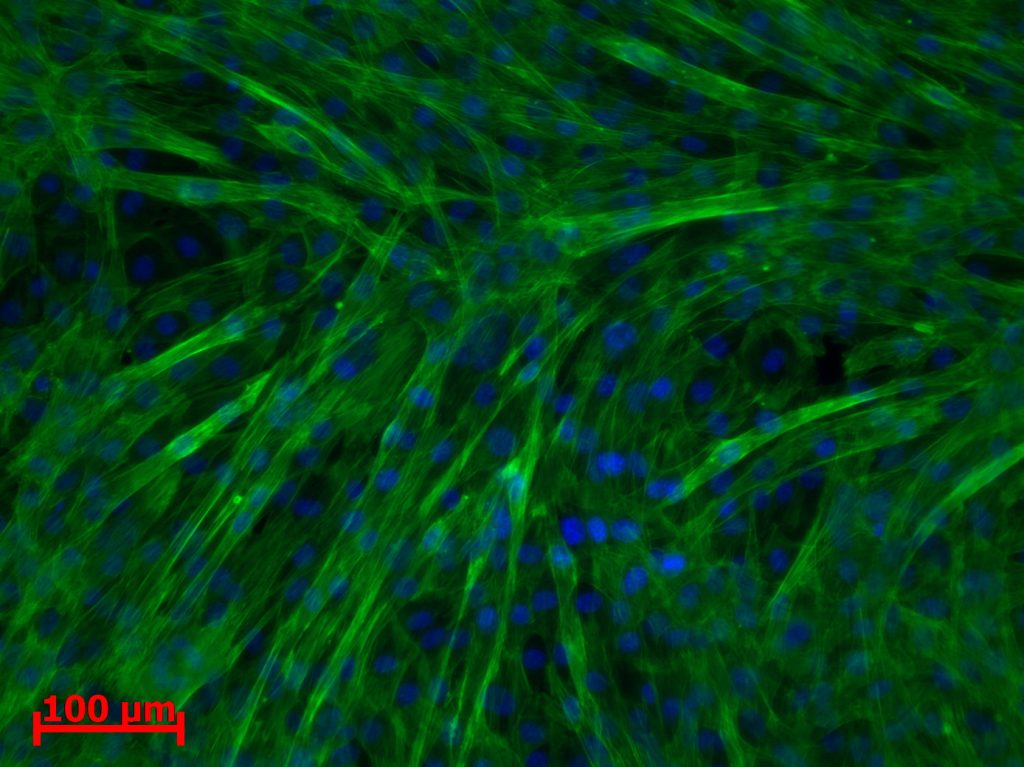
A device was designed and implemented to perfuse adherent cell cultures with culture medium for prolonged periods of time on the RPM. Computations showed that a low flow rate (< 1 ml/h) does not negatively affect cell culture behavior. This was confirmed by an experiment where mouse myoblasts were exposed for 24 hours to the low medium perfusion. Finally mouse myoblasts were allowed to differentiate under simulated microgravity for six days and samples were taken every second day. The cells were stained with DAPI and Rhodamine-Phalloidin and subsequently analyzed by fluorescent microscopy. Myoblast differentiation was determined by factors such as cell density, the ratio of fused cells, the area coved by myotubes, the area covered by fibrous structures, the myotube width and by myotube orientation.
Myoblast exposed to perfusion for one day showed no change in morphology or cell alignment. In comparison to a non-perfused control, proliferation was enhanced through the improved nutrition situation. Myoblasts differentiating under simulated microgravity showed no enhanced or impeded differentiation. Proliferation was reduced in all three experiments and myotube width was unaffected.
The finding on reduced proliferation on the RPM is in agreement with previous studies. The finding on unchanged differentiation disagrees with a previous study carried out with rat myoblast differentiating on the RPM. Myoblasts were found to have differentiation significantly disturbed. In two other studies mouse myoblasts were exposed to two-dimensional clinostat rotation. Clinostat also simulates microgravity, but rotate around one horizontal axis. At first differentiation appeared to be reduced, but was non-significantly reduced after the hardware was changed to cause less shear stress. However, the results among all of the studies are not directly comparable due to differences in biological models, hardware and differentiation measuring method. Observations during this project suggest that shear stress caused through entrapped air bubbles disturb differentiation.